GlnK proteins
PII proteins constitute a ubiquitous and highly conserved family of signaling proteins in prokaryotes and plants. They integrate metabolic status information by monitoring and responding to the cellular levels of 2-oxoglutarate and glutamine as the substrate and product of ammonium assimilation though the GS/GOGAT system, as well as to the ratio of ATP and ADP as an indicator of the cellular energy level. PII proteins thus are key regulators for the assimilation of the essential element nitrogen. They invariably form homotrimers and act predominantly through direct interaction with a variety of target proteins such as the Amt proteins.
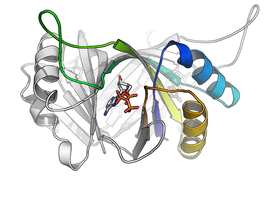
The hyperthermophilic archaeon Archaeoglobus fulgidus contains three GlnK proteins, functionally associated to ammonium transport proteins (Amt). We have characterized GlnK2 and its interaction with effectors by high-resolution X-ray crystallography (PDB codes 3NCP, 3NCQ, 3NCR) and isothermal titration calorimetry. Binding of adenosine nucleotides resulted in distinct, cooperative behavior for ATP and ADP. While 2-oxoglutarate has been shown to interact with other GlnK proteins, GlnK2 was fully insensitive to this key indicator for a low intracellular nitrogen level. These findings point towards different regulation and modulation patterns and add to our understanding of the flexibility and versatility of the GlnK family of signaling proteins.