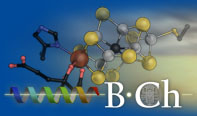
Nitrous Oxide Reductase

Nitrous oxide is a potent greenhouse gas as well as an ozone-depleting substance that was recently named the most critical climate agent of the 21st century. It is released through natural processes, but also - and increasingly - from anthropogenic sources in industrial production and agriculture: The reduction of nitrous oxide to dinitrogen is the final step of the bacterial pathway of denitrification, an efficient energy metabolism consisting of the four-step reduction of nitrate via nitrite, nitric oxide and nitrous oxide to dinitrogen. With this, soil bacteria compete with crop plants for the nitrate used as a fertilizer, to the effect that approximately half the nitrogen fertilizer brought out in fields is lost to denitrification. Of the denitrificatory enzyme, nitrous oxide reductase is the most sensitive to atmospheric dioxygen, so that it is the most frequent to fail, resulting in the release of significant amounts of nitrous oxide to the atmosphere.
Similar to the case of nitrogenase, only one single enzyme is known to catalyze the reduction of nitrous oxide. The reductase is a dimeric copper protein of 130 kDa in mass, containing two unusual metal sites. The binuclear CuA center is highly similar in structure to the site of the same name found in respiratory cytochrome c oxidase. The second metal site, however, is entirely unique to nitrous oxide reductase. The tetranuclear copper center CuZ is a [4Cu:2S] cluster and the site of substrate binding in the enzyme. We were able to elucidate the access pathways and binding mode for nitrous oxide at this cluster, providing first insight into the mechanism of activation and reduction of the inert small molecule N2O at the large metal cluster.
References:
- Wüst, A., Schneider, L., Pomowski, A., Zumft, W. G., Kroneck, P. M. H. & Einsle, O. (2012) Nature’s way of handling a greenhouse gas: The copper-sulfur cluster of purple nitrous oxide reductase. Biol. Chem., 393, 1067-1077.
- Pomowski, A., Zumft, W.G., Kroneck, P.M.H. & Einsle, O. (2011) N2O binding at a [4Cu:2S] copper-sulphur cluster in nitrous oxide reductase. Nature, 477, 234-237.
- Pomowski, A., Zumft, W.G., Kroneck, P.M.H. & Einsle, O. (2010) Crystallization of purple nitrous oxide reductase from Pseudomonas stutzeri. Acta. Crystallogr., F66, 1541-1543.
- Einsle, O. & Kroneck, P. M. H. (2004) Structural Basis of Denitrification.
Biol.Chem., 385, 875-883.
- Simon, J., Einsle, O, Kroneck, P. M. H. & Zumft, W.G. (2004) The unprecedented nos gene cluster of Wolinella succinogenes encodes a novel respiratory electron transfer pathway to cytochrome c nitrous oxide reductase. FEBS Lett., 569, 7-12.